Hydrogen is also part of the Activity Series When Hydrogen is part of an acid it. A BC - AC B Where A kicks out B and takes its place A B C - AC Try some.

Grand Official Opening Of Fresh N Marine Flagship Store On 1 January 2018 Good Environment Grands Marine
The reaction takes place when different types of metals or halogens come into contact with one another such as zinc dissolving in an acidic solution.

. One reactant breaks apart to form new compounds. The Single Replacement Reaction is a type of chemical reaction that occurs when one element replaces another in a compound. A single-replacement reaction also called a single-displacement reaction is one in which a pure element and a compound react chemically so that the products include another pure element and a different compound via exchanging of two species.
ZnCuCl2 Cu ZnCl2. Single and Double Replacement Reactions Study concepts example questions explanations for AP Chemistry. Up to 10 cash back AP Chemistry.
One reactant breaks apart to form new compounds. Two reactants combine to form one product. A single-replacement reaction replaces one element for another in a compound.
A metal only replaces a metal and a nonmetal only replaces a nonmetal. In a single replacement reaction a single element replaces an atom in a compound producing a new compound and a pure element. Two reactants exchange elements with each other.
Learn with flashcards games and more for free. One reactant replaces an element in another reactant. Single replacement reaction displacement.
This means single replacement reactions are by definition oxidation-reduction reactions. A single-displacement reaction is a chemical reaction where one reactant is exchanged for one ion of a second reactant. They bubble and hiss.
Displacement reactions can be further classified into single replacement reaction and double replacement reaction. And then will perform several single replacement reactions of their own. A single-displacement reaction occurs when an element replaces another element in a compound.
Alright so were going to talk about single replacement reactions you might also. A single replacement reaction occurs when an element reacts with a compound displacing another element in that compound according to the University of Memphis. Its the reactions that are the impressive part.
There are two types of single replacement reactions. One reactant replaces an element in another reactant. Like double replacement reactions metals always replace metals and nonmetals always replace nonmetals in a compound.
Two reactants combine to form one product. A single replacement reaction occur when an element in a substance is replaced by other element. What happens in a single-replacement reaction.
One of the reactions can even be forced to make a rather loud pop. A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. Helpful 0 Not Helpful 1 Add a Comment.
A halogen replaces another halogen that is in solution. Instant Classic -For Reactions always write the compound first followed by the free element. A double-replacement reaction exchanges the cations or the anions of two ionic compounds.
Single displacement reactions take the form. A metal single replacement reaction looks like this in equation form. What happens in a single-replacement reaction.
A metal replaces another metal that is in solution. It is also known as a single-replacement reaction. 6 Diagnostic Tests 225 Practice Tests Question of the Day Flashcards Learn by Concept.
For example in the cation replacement reaction A gains electrons and C loses electrons. All AP Chemistry Resources. The periodic table or an activity series can help predict whether single-replacement reactions occur.
Two reactants exchange elements with each other. Two reactants yield two products. An example of this reaction is when potassium K reacts with water H2O it formed a colorless solid compound named potassium hydroxide KOH and hydrogen gas H2 is set free.
Only a more reactive element. In a single replacement reaction or single displacement reaction a single uncombined element replaces another in a compound. In single replacement reactions one ion or an atom of an element is displaced by another ion or atom discussed in detail below.
To drive this point home well assign oxidation states in some of our examples to follow the flow of electrons in the reaction. Definition of single replacement or single displacement reactions. ABC B AC.
Learn what a single replacement reaction is how to read an activity series to predict what will happen in a single replacement reaction. In other words it is where an element reacts with a compound and replaces one component of the compound. Predicting and determining the products using the reactivity series.
In a double replacement reaction there is interchanging of ions or atoms between the reactants. Create An Account Create Tests Flashcards.

Amine Chemical Compound Britannica
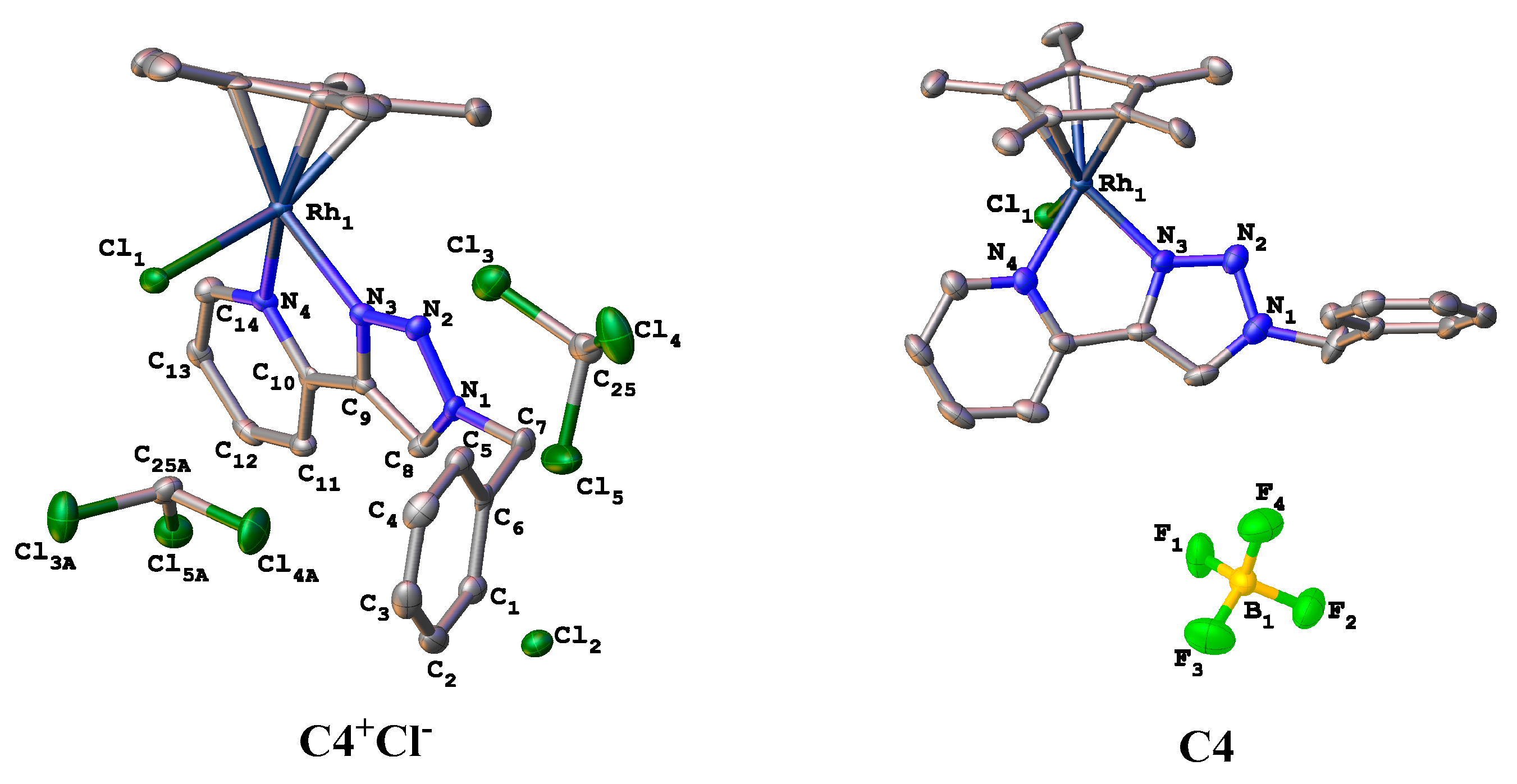
Molecules Free Full Text New Triazolyl N N Bidentate Rh Iii Ir Iii Ru Ii And Os Ii Complexes Synthesis And Characterization Probing Possible Relations Between Cytotoxicity With Transfer Hydrogenation Efficacy And Interaction With Model

Inducing Single Handed Helicity In A Twisted Molecular Nanoribbon Journal Of The American Chemical Society

Blue Titanium Steel Ring Personality Domineering Ring Etsy Rings For Men Blue Rings Steel Ring

What Is The General Form Of A Single Replacement Reaction Brainly Com

4 2 Types Of Chemical Reactions Single And Double Replacement Reactions Chemistry Libretexts
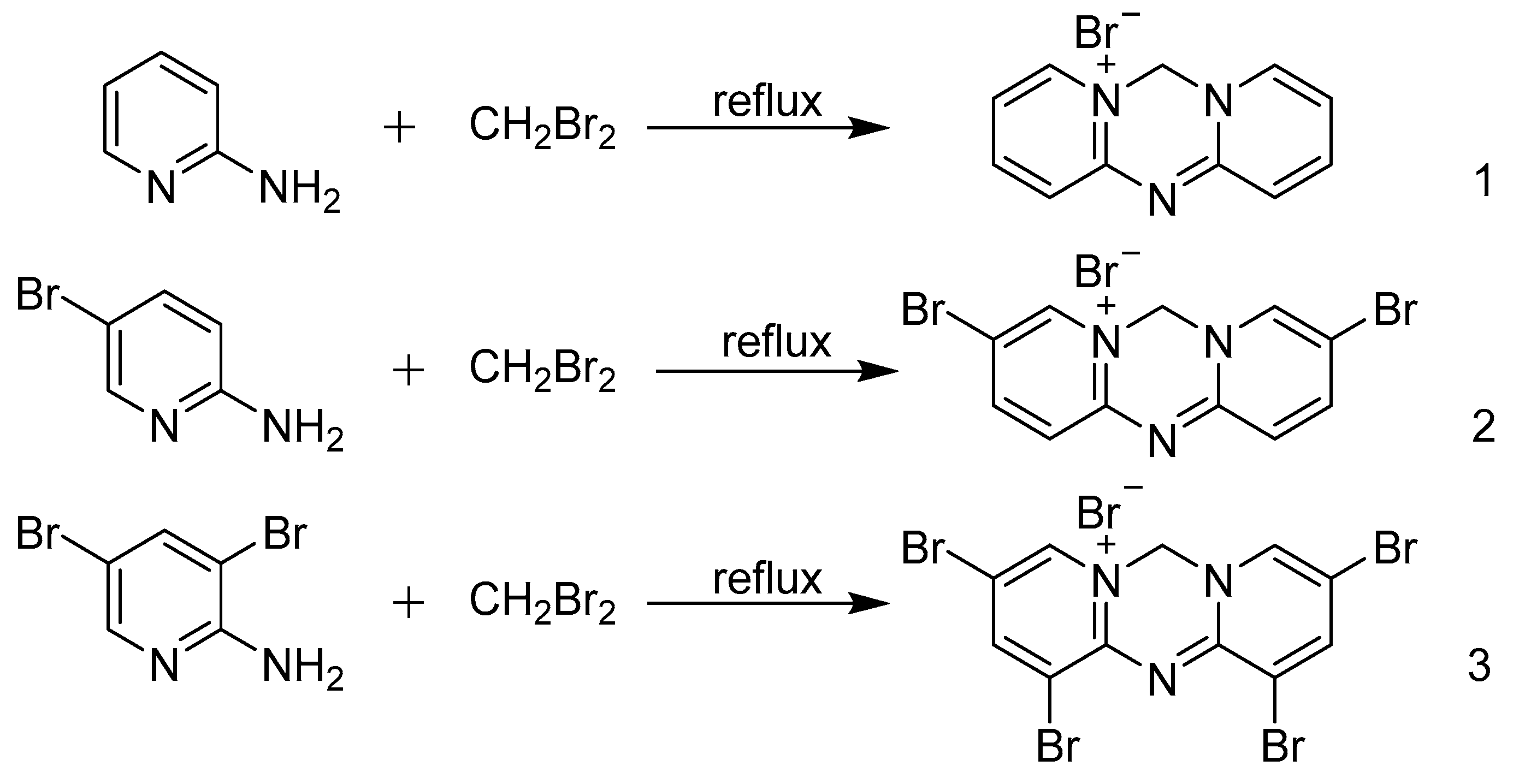
Materials Free Full Text Solvent Free And Efficient One Pot Strategy For Synthesis Of The Triazine Heterocycle Azacyanines Html
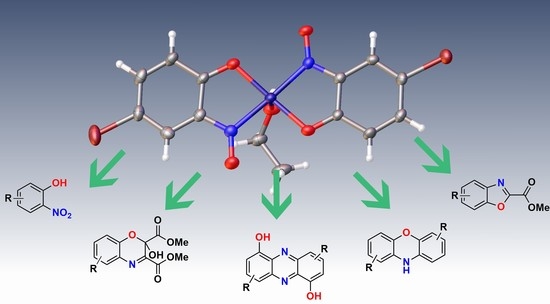
Molecules Free Full Text The Synthesis And Utility Of Metal Nitrosophenolato Compounds Highlighting The Baudisch Reaction Html

An Expeditious Benzannulation Reaction Of Indol 3 Yl But 3 Yn 2 Ols To Substituted 2 Iodocarbazoles Via Domino 5 Endo Spirocyclization Selective Vinyl Shift And Aromatization Yaragorla 2019 European Journal Of Organic Chemistry Wiley Online Library
0 Comments